Visible light disinfection is a lighting industry innovation that allegedly inactivates pathogens, such as bacteria and viruses, with violet light. Unlike germicidal lamps emitting ultraviolet-C radiation, visible light does not pose the considerable risk of photokeratitis (“snow blindness”) or erythema (“sunburn”) for room occupants. With the availability of inexpensive and efficient violet LEDs, major luminaire manufacturers are adopting the technology for everything from hospital operating theaters to residential kitchens.
As is often the case with new technologies, the marketing efforts of both large and small manufacturers sometimes exceed the bounds of scientific evidence. For example, one manufacturer claims that its products are “scientifically proven to kill 94% of SARS-CoV-2 under a range of conditions,” without any reference to supporting academic literature.
Other manufacturers reference peer-reviewed medical literature, but the details can be vague. One study examined the efficacy of visible light disinfection in operating theaters where the manufacturer of the luminaires “provided technical assistance before and during installation to ensure that proper illumination and disinfecting dose was achieved across the entire [operating room].” Unfortunately, the researchers did not specify the disinfecting dose, or the irradiance multiplied by exposure period.
From an engineering perspective, this is both frustrating and irresponsible. If a professional lighting designer were asked to design or specify a visible light disinfection system in any setting, they are responsible for understanding both the benefits and risks of the technology, and for ensuring appropriate target irradiance levels and operating conditions. Unfortunately, no lighting or healthcare industry standards or guidelines exist to date for designers to follow. ASHRAE, IEC, IES, IUVA, and other organizations are currently developing ultraviolet radiation standards and recommended practices.
How visible light disinfection works
Visible light disinfection is by no means a new discovery. The ancient Egyptians reported the health benefits of sun exposure approximately 6 millennia ago, while ancient Greek, Roman, and Arabic cultures similarly recognized the therapeutic values of sunlight, or heliotherapy. More recently, Niels Ryberg Finsen was awarded the 1903 Nobel Peace Prize in Medicine for his discovery that optical radiation from electric arc lamps (phototherapy) was an effective treatment for lupus vulgaris, a painful and debilitating bacterial skin infection (Fig. 1, above).
But how does it work? Germicidal lamps — which include low-pressure mercury vapor arc lamps emitting 254-nm UV-C radiation, Kr-Cl excimer lamps emitting 222-nm UV-C radiation, and LEDs emitting narrowband UV-B or UV-C radiation — disrupt the DNA of bacteria and fungal spores, and the DNA or RNA of viruses. This disables the cellular mechanisms of bacteria and fungal spores, inactivating them, and prevents viruses from being able to replicate.
Individual visible light photons do not present sufficient energy to disrupt DNA or RNA. Most bacteria, fungi, and protozoa contain intracellular (endogenous) porphyrins that strongly absorb visible light in a region of the spectrum called the Soret band (Fig. 2). The prevailing theory states that these photosensitizers consequently transfer the photon’s energy to an oxygen molecule within the cell, producing a reactive oxygen species (ROS) molecule, such as singlet oxygen or hydrogen peroxide, that inactivates the cell by disrupting its cellular machinery.
Evidence exists for other explanations of how visible light inactivates pathogens, including different photosensitizers, such as riboflavin acting at 450 nm and different cellular inactivation mechanisms.
Unlike bacteria, fungi, and protozoa, viruses do not contain porphyrins. As such, they theoretically should not be susceptible to blue light wavelengths. Nevertheless, growing evidence suggests that they may be.
Most visible light disinfection studies to date have focused on the spectral region of 385 to 420 nm, with 405 nm shown to be the most effective. However, bactericidal effects of visible light have been demonstrated with wavelengths up to 740 nm1. These are presumably due to the absorption characteristics of different endogenous photosensitizers, such as riboflavin, around 450 nm. However, the dose required for inactivation generally increases exponentially with wavelength.
Various photosensitive drugs are used in photodynamic therapy, wherein the drugs are absorbed by the diseased cells and then activated by exposure to visible light. This topic is relevant to visible light disinfection in that the U.S. Food and Drug Administration may choose to regulate the use of 405-nm light as a potential photosensitizer, particularly in hospitals where patients may be administered medications with known photosensitizing effects.
Visible light disinfection has a firm scientific basis for medical applications. However, its usefulness in disinfecting enclosed architectural spaces is more nuanced. The remainder of this article focuses on considerations for evaluating or designing with visible light disinfection products for different applications.
Irradiance and dose
The first step to determining what irradiance and dose of visible light are needed to achieve satisfactory results is to define our terms of measurement. Irradiance, measured in milliwatts per square centimeter (mW/cm2), is the radiant equivalent of illuminance, which can be measured in lumens per square centimeter. Dose is the irradiance multiplied by the exposure time in seconds. Expressed in joules (i.e., watt-seconds) per square centimeter (J/cm2), dose is the radiant equivalent of luminous energy, but most lighting designers have never needed to consider this unit.
Next, we must understand what it means to “inactivate” pathogens in a practical sense. When exposed to constant irradiance, whether visible light or ultraviolet radiation, the rate of inactivation is initially exponential. For example, if 90% of the pathogens are inactivated after one hour, 90% of the surviving pathogens will be inactivated after another hour. Thus, there will be 90% inactivation after one hour, 99% after two hours, 99.9% after three hours, and so on.
It is often more convenient to express these numbers in log10 units, where one log10 unit represents 90% inactivation of the initial number of pathogens, two log10 units represents 99%, three log10 units 99.9%, and so on. Another term for log10 units is D-value.
The desired degree of disinfection depends on the application. For example, the FDA requires sterilized surgical instruments to have D-values of 6 or greater (99.9999% inactivation), on the assumption that the remaining pathogens are too few in number to cause infections. However, for visible light disinfection, specifying 90%, or one log10, inactivation is common.
Reducing the number of pathogens by 90% may not seem useful, but visible light disinfection — like UV-C disinfection in a hospital — should always be considered as a supplement to terminal cleaning. Its primary purpose is not to eradicate existing bacterial colonies, but to prevent those colonies from growing before the next terminal cleaning.
Pathogen species and variants
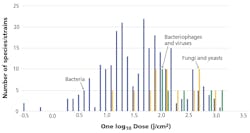
Some luminaire manufacturers list numerous pathogens as susceptible to their products — Staphylococcus aureus, Listeria monocytogenes, Salmonella enteritidis, among others. Fewer than 100 bacteria species have been examined for visible light susceptibility and even fewer fungi and viruses. Even among this select group of common pathogens, the range of visible light doses needed to achieve 90% inactivation can vary significantly (Fig. 3). For example, genetic variants of methicillin-resistant Staphylococcus aureus (MRSA) may need between 13.7 and 1200 J/cm, depending on laboratory conditions. In general, observed dose differences for single species of up to one order of magnitude may be expected from different laboratory studies, with fungi and viruses requiring considerably higher doses than most bacteria.
For test purposes, pathogens can be grown as colonies on growth media in petri dishes or in solution, or aerosolized for airborne species. Their susceptibility to visible light may depend on the irradiance, exposure time, relative humidity, growth medium, oxygen concentration, density (expressed in colony-forming units per milliliter, or CFU/ml), and pathogen strain.
Other uncertainties exist. When a paper reports a given irradiance, it could be due to a single LED with a range of 10:1 over the irradiated area. For LED arrays, the irradiance could be measured at the sample surface or the LED diffuser plate. The transparent petri dishes could be placed on a reflective base, which could increase and possibly double the irradiance the pathogens receive due to reflected light. Growth medium additives can include phenol red, which strongly absorbs in the blue/violet region of the spectrum. This makes it difficult to compare studies of minimum dose needed to achieve one log10 inactivation.
To further complicate matters, bacteria may form biofilms on surfaces to protect themselves from desiccation, antibiotics, the host body’s immune system, and other environmental factors. Biofilms are found everywhere. The films themselves are not alive, but they may protect bacteria from being inactivated by ultraviolet radiation, particularly 222-nm UV-C. (One of the arguments in favor of far-UV is that the short wavelengths are unable to significantly penetrate corneal and skin cells to cause DNA damage. The same argument may apply to organic biofilms. However, it has been demonstrated that 405-nm visible light can effectively treat some bacteria species encapsulated in biofilms.
Impact of blue and white light on microbes
In any microbial population, a fraction of pathogens will be resistant to UV radiation. Once 99% of the population has been inactivated, it is not uncommon for the rate of inactivation to decrease significantly. In other words, the surviving pathogens require higher doses to inactivate them. The same principle applies to bacteria developing resistance due to the overuse of antibiotic drugs.
Fortunately, as stated earlier, visible light disinfection by design does not seek to eradicate microbial populations but to limit their growth or at best achieve 90% inactivation. Therefore, it is unlikely that pathogens will develop blue light resistance, as they have little evolutionary pressure to do so. Indeed, studies with sublethal doses of blue light have shown that the bacteria do not develop blue light resistance. However, at least one counter-example with Staphylococcus aureus has been reported.
One concern is that laboratory studies typically isolate the pathogens being studied from environmental factors, including ambient lighting where white light illumination may actively encourage bacterial and fungal colony growth. How should such studies be interpreted in real-world conditions where pathogens are exposed to both violet light for disinfection and ambient white light for illumination? This will be discussed in a follow-on article on the practice of visible light disinfection.
Some manufacturers promote visible light disinfection at irradiance levels where the exposure time is a minimum of eight hours to achieve 90% inactivation in the laboratory. If the ambient illumination promotes colony growth, this may reduce the effectiveness of the visible light disinfection. One study3 using 5600K white-light LEDs (which exhibit a significant peak at approximately 450 nm due to the blue pump LEDs) required illuminance of 2,400 lx over 34 hours to achieve a 90% reduction of Staphylococcus sp. bacteria on glazed stoneware. Adding 2.5 mW/cm2 of 405-nm irradiation reduced this to 3.5 hours, but the visual appearance of the illumination was well off the Planckian locus and decidedly purplish in color.
Viruses and bacteriophages
Research continues into whether viruses and bacteriophages (viruses that infect bacteria) are susceptible to visible light. Scientists have argued that viruses and bacteriophages should not be susceptible because they do not contain porphyrins or other endogenous photosensitizers that can generate ROS. However, viruses examined in the laboratory may be suspended in organically rich growth media, such as fetal bovine serum, which can form ROS. Also, the host cells may contain endogenous photosensitizers. Alternatively, they may become inactivated due to upregulated production of damaging proteins by bacteriophages.
Some studies have examined viruses in phosphate-buffered saline, which rules out endogenous photosensitizers. However, these studies have been mostly limited to enveloped viruses, which include coronaviruses such as SARS-CoV-2, where visible light possibly damages the lipids of the envelopes rather than the virus DNA or RNA5.
Evidence exists that visible light is capable of inactivating viruses directly, but the mechanisms are still under investigation. Riboflavin in solution may have an impact on photoinactivation results, and virus reduction with visible light may require considerably higher doses under other conditions, such as in air2,4.
Discussion
Admittedly, this information may seem far removed from the concerns of day-to-day lighting design, but it has significance. When a manufacturer claims that its visible light disinfection luminaires are “scientifically proven to kill 94% of SARS-CoV-2 under a range of conditions,” it is necessary to define that “range of conditions.”
Expecting luminaire manufacturers to have full knowledge of the science behind visible light disinfection is unreasonable, and they are likely not being intentionally deceptive. However, lighting professionals who design and specify visible light disinfection systems have a clear ethical and possibly legal responsibility. This begins with understanding how the technology works. The second installment of this article will examine the practice of visible light disinfection, with an emphasis on whole-room disinfection applications.
Information statement
All of the statements made in this article are supported by peer-reviewed academic studies. An extended version of this article with 54 references is available from the IES Forum for Illumination Research, Engineering, and Science (FIRES) .
References
1. M. Hessling et al., “Photoinactivation of Bacteria by Endogenous Photosensitizers and Exposure to Visible Light of Different Wavelengths – A Review on Existing Data,” FEMS Microbiology Letters, 367 (2017).
2. M. Hessling et al., “Review of Virus Inactivation by Visible Light,” Photonics, 9:113 (2022a).
3. M. Hessling et al., “Surface Disinfection with White-Violet Illumination Device,” AIMS Bioengineering, 92:93–101 (2022b).
4. W. Kowalski, Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection, New York, NY: Springer (2009).
5. R. Rathnasinghe et al., “The Virucidal Effects of 405 nm Visible Light on SARS-CoV-2 and Influenza A Virus,” Scientific Reports, 11(11):19470 (2021).
6. R.M. Tomb et al., “Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light,” Photochem. and Photobio., 94(3):445–-458 (2018).
This online article has been expanded with text, references, and images — abridged version published in the July/August 2022 issue of LEDs Magazine.
Get to know our expert
IAN ASHDOWN, P. Eng. (Ret.), FIES, is a consultant with 40 years’ experience in lighting design, research and development, and software engineering. He is currently senior scientist and president of SunTracker Technologies in Victoria, British Columbia, Canada, where he is developing lighting design software for architectural, horticultural, entertainment, and health applications. Ashdown has presented at industry events and contributed, reviewed, and edited several technical journals and trade publications.
For up-to-the-minute LED and SSL updates, follow us on Twitter. You’ll find curated content and commentary, as well as information on industry events, webcasts, and surveys on our LinkedIn page and our Facebook page.

Ian Ashdown | President & Owner
Ian Ashdown is a consultant with nearly 40 years' experience in lighting design, research and development, and software engineering. He is chief senior scientist for SunTracker Technologies. He is currently hard at work on several projects involving intelligent lighting controls and real-time dynamic lighting design software.




![The DesignLights Consortium continues to make progress in shifting outdoor lighting products and implementation practices toward a more restrained and thoughtful strategy. [Image does not represent a DLC qualified fixture.] The DesignLights Consortium continues to make progress in shifting outdoor lighting products and implementation practices toward a more restrained and thoughtful strategy. [Image does not represent a DLC qualified fixture.]](https://img.ledsmagazine.com/files/base/ebm/leds/image/2024/08/66be810888ae93f656446f61-dreamstime_m_265700653.png?auto=format,compress&fit=&q=45&h=139&height=139&w=250&width=250)
