INTERVIEW: Polarity Medical knows how to ‘handle’ infections
Polarity Medical Technology is not the typical “lighting industry” company that LEDs Magazine tends to cover. In fact, it isn’t a lighting company at all but a developer of UV-C disinfection technologies taking an unconventional approach to high-touch sanitization.
As LEDs has reported multiple times, although studies later demonstrated that the virus responsible for the COVID-19 pandemic was mainly transmitted via airborne particles, there are plenty of other microbes, spread by surface contact, which affect those with vulnerable immune systems — especially in the clinical environment. And although we have covered some surface-disinfection products, they were frequently targeted toward high-touch mobile or personal device sanitization and have mainly waned in popularity.
As Polarity Medical Technology CEO Dr. Tom Chi — professionally trained in Traditional Chinese Medicine — pointed out, the U.S. Centers for Disease Control and Prevention (CDC) claims that about 1 in 31 patients in the U.S. healthcare system acquires a healthcare-associated infection (HAI) each day.
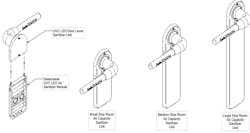
When Chi reached out to discuss Polarity’s use of UV-C LEDs (employing Crystal IS Klaran technology) in its SafeTOUCH devices, our initial question was “why did your company engineer a door handle that safely sterilizes after being touched?” The second was “how does one know that this operates safely?” And of course we wanted to understand how a door handle could make a difference in protecting people from common infectious agents.
LEDs Magazine: Can you briefly explain your company’s origins and what led to developing the SafeTOUCH sterilization devices for door handles?
Tom Chi: Originally when my mother got COVID-19 early in the pandemic, I read that half of an office staff becomes infected from one germy doorknob in just 4 hours. As I researched and worked on these designs, it became clearer that COVID-19 was not likely transmitted easily by touch but instead by air. Yet I noted that the major transmission point for HAIs was indeed on patient-room door levers and that about 1 million people die each year worldwide from HAIs. As many have heard, MRSA [methicillin-resistant Staphylococcus aureus] is one of the biggest problems in healthcare, and other antibiotic-resistant microbes are on the rise.
With our devices, we can eliminate perhaps 80% of transmission concerns in hospitals and project that initially tens of thousands of people per year will be spared this demise. With full assimilation of the door handle technology, we should see those numbers increase to the hundreds of thousands spared. Plus, at any given time over 1 million people are affected by HAIs who do not die; and they too can be spared illness and the $200 billion in mitigation costs per year.
LEDs: When we first learned of the SafeTOUCH door handles, our first question was about potential risks from using UV-C technology emitted around the door handle — a high-touch area, as you have mentioned. How is the LED technology implemented to prevent a risk of skin or eye injury to people in the vicinity?
Chi: First, you should know the SafeTOUCH UV-C door levers are designed to destroy pathogens in 3 to 6 seconds using a smaller number of UV-C LEDs that reduces peak power required by nearly 88%, which means a compact, long-life battery is sufficient to power the device for up to 100,000 cycles per year. It can also be wired or connected with a 9V wall-wort plugged directly to an outlet if desired.
We use a structurally reinforced quartz touch surface that enables the UV-C radiation to be emitted only within a small local area on the door handle, without other equipment or housings external to the handle that can cast shadows where the UV would be ineffective. The proprietary rotating mechanism ensures an even emission of UV radiation to the surface.
No power is activated to the UV sources on either side of the door and jamb until the door closes and the infrared proximity sensor detects that someone has moved away from the unit. Also, it’s important to note the inverse square law — the amount of radiation emitted to disinfect the handle diminishes by the time it irradiates the outer surface of the quartz door lever.
LEDs: You started with the SafeTOUCH door handle, which is a direct replacement installable on any standard door. Have you expanded your range?
Chi: After SafeTOUCH, we developed SafeAIRE add-on modules which destroy airborne pathogens with continuous air circulation, also installed right at the door lever; the SmartKILL programmable user interface; and SafeSPHERE, which combines both the UV-C door levers and airborne modules with options for Bluetooth or Wi-Fi extenders. It can also include audio/video and emergency call buttons or access requests in one integrated solution — all of this easily installable by building technicians, facilities engineers, or general electrical personnel.
LEDs: Can you share with us how many units have been installed so far, or number of customers that have employed the SafeTOUCH handle technology?
Chi: We currently have orders for two hospital installations in the Netherlands and we are finalizing details and timelines for the fourth quarter of 2023. After that, it should be exponential. The fact that these devices meet Minamata Convention requirements for eliminating mercury components, and are FDA, EPA, and CE compliant, helps us start conversations with large providers like Assa Abloy, a major supplier of access hardware. And we are in a collaboration with Assa Abloy at this time.
Hospitals are our major priority. There are an estimated 187,500 facilities globally! We’ve also been approached by a major cruise line for cabin door and common-area placement. Hotels should also be interested in adding these devices as they are similar in cost to existing architectural hardware. Finally, we envision a lot of corporate office and retail usage and will soon launch for B2C on Amazon and Alibaba and in Home Depot, Walmart, and potentially other retailers.
LEDs: Do you have patents and plans for leveraging your IP in the marketplace?
Chi: We have multiple patents pending and most of our claims have been validated for our international patents by the USPTO International Searching Authority.
I don’t think I can overstate that this is a breakthrough, but also something that we take very seriously as a disruptive product. Signify, GE Medical, and Ushio all hoped to create similar devices and we happened upon the combination of solutions that solve the conundrum of cost, peak power, 100,000 cycles-per-year battery, a true 12VDC system with full irradiance, and thermal challenges for these form factors.
There is definite potential for directing use of our IP in the marketplace, and we’ll consider those opportunities along the way.
One fun fact: Did I mention that our contract engineers created the sophisticated light saber for Disney? We have excellent engineering resources and this is only the beginning. We anticipate being a “unicorn” company within 4 years with just 2.5% of hospitals using these devices.
Learn more about Polarity Medical’s SafeTOUCH from a short video on the company’s homepage.
SafeTOUCH is a registered trademark of Polarity Medical Technology.
This interview has been edited and condensed for clarity.
CARRIE MEADOWS is managing editor of LEDs Magazine, with 20-plus years’ experience in business-to-business publishing across technology markets including solid-state technology manufacturing, fiberoptic communications, machine vision, lasers and photonics, and LEDs and lighting.
Follow our LinkedIn page for our latest news updates, contributed articles, and commentary, and our Facebook page for events announcements and more. You can also find us on Twitter.

Carrie Meadows | Editor-in-Chief, LEDs Magazine
Carrie Meadows has more than 20 years of experience in the publishing and media industry. She worked with the PennWell Technology Group for more than 17 years, having been part of the editorial staff at Solid State Technology, Microlithography World, Lightwave, Portable Design, CleanRooms, Laser Focus World, and Vision Systems Design before the group was acquired by current parent company Endeavor Business Media.
Meadows has received finalist recognition for LEDs Magazine in the FOLIO Eddie Awards, and has volunteered as a judge on several B2B editorial awards committees. She received a BA in English literature from Saint Anselm College, and earned thesis honors in the college's Geisel Library. Without the patience to sit down and write a book of her own, she has gladly undertaken the role of editor for the writings of friends and family.
Meadows enjoys living in the beautiful but sometimes unpredictable four seasons of the New England region, volunteering with an animal shelter, reading (of course), and walking with friends and extended "dog family" in her spare time.





![The DesignLights Consortium continues to make progress in shifting outdoor lighting products and implementation practices toward a more restrained and thoughtful strategy. [Image does not represent a DLC qualified fixture.] The DesignLights Consortium continues to make progress in shifting outdoor lighting products and implementation practices toward a more restrained and thoughtful strategy. [Image does not represent a DLC qualified fixture.]](https://img.ledsmagazine.com/files/base/ebm/leds/image/2024/08/66be810888ae93f656446f61-dreamstime_m_265700653.png?auto=format,compress&fit=&q=45&h=139&height=139&w=250&width=250)
